Evaluation of Interfacial Tension Using DPD
Dissipative Particle Dynamics (DPD) was used to compute an interface model of water and octane. Based on solubility parameters (SP values) obtained from Full-Atomistic Molecular Dynamics (FAMD) for each component, Flory–Huggins χ parameters were estimated, and DPD interaction parameters were set. Interfacial tension was evaluated from stress values in each direction, showing good agreement with experimental results.
Use Cases Highlights
- Evaluation of interfacial tension using DPD
- Determination of interaction parameters from solubility parameters
- Agreement with experimental values
Construction of interfacial models
An interface formed by 3,000 water and 1,000 octane DPD particles is shown. The interface remains after relaxation. The J-OCTA modeling function allows easy construction.
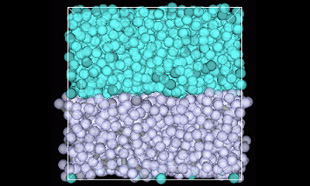
Interface model of water and octane
Evaluation of interfacial tension and comparison with experiments
Interfacial tension was calculated from stress values in each direction using stress data. Tracking time variations, the average value was evaluated as 50.1 [dyn/cm], showing good agreement with the experimental value of 51.7 [dyn/cm].
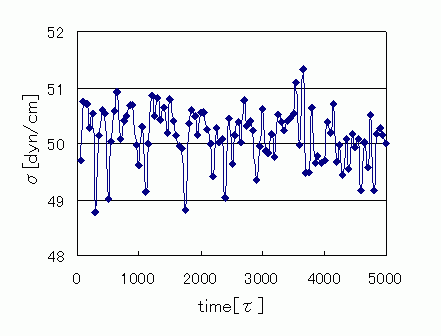
Time variation of interfacial tension
Change in interfacial tension when interactions are varied
The interfacial tension was evaluated by varying the particle interaction parameter aij in DPD calculations. Since interactions in DPD represent repulsion, stronger interactions resulted in increased interfacial tension.
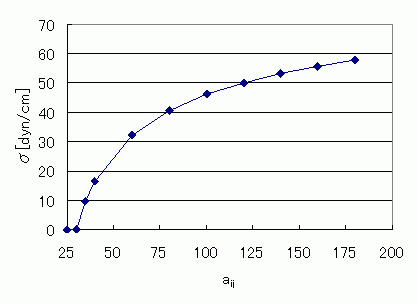
Relationship between interfacial tension and interaction parameter aij
Reference
[1] A.Maiti and S. McGrother, J. Chem. Phys., 120, 3, 15 (2004)
Details of analysis
Inquiries Regarding Products
Have questions about product implementation? Contact us today.