Solubility for water
The free energy perturbation (FEP) method implemented in the Molecular Dynamics engine GENESIS (developed mainly by RIKEN) was used to calculate the hydration free energy of five small molecules, including methanol and caffeine. The results showed good agreement with experimental values, confirming that quantitative evaluation of solubility is possible. This is a useful method for molecular design in pharmaceuticals and cosmetics.
Use Cases Highlights
- Evaluation of solvation free energy using the free energy perturbation method
- Capability for high-accuracy evaluation
Evaluation of hydration free energy using the free energy perturbation method
The procedure for evaluating solvation free energy using the Free Energy Perturbation (FEP) method is shown. The free energies in vacuum and in water are calculated through the “deletion” process of the solute, and the solvation free energy is obtained from their difference.
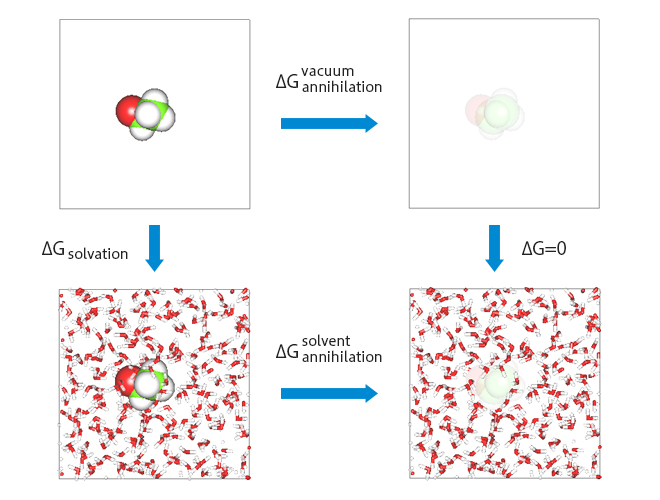
Operational procedure for evaluating solvation free energy
Capability for high-accuracy evaluation
A comparison graph of calculated solvation free energies for five solutes with experimental values from literature is shown. The calculated values agree well with the experimental values, confirming the validity of the method.
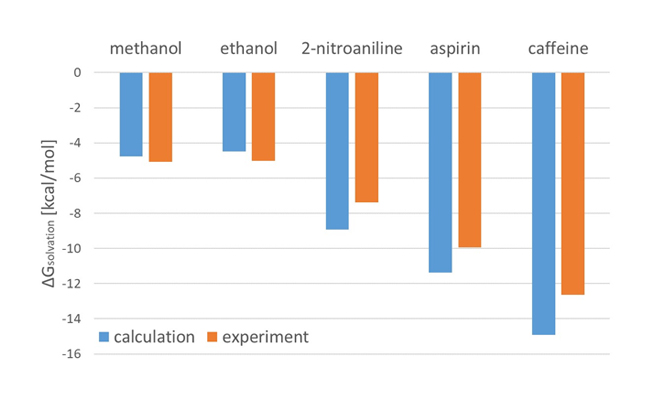
Comparison of calculated and experimental hydration free energies for each solute
Reference
[1] C. Kobayashi, J. Jung, Y. Matsunaga, T. Mori, T. Ando, K. Tamura, M. Kamiya, and Y. Sugita, J. Compute. Chem. 38, 2193-2206 (2017). https://doi.org/10.1002/jcc.24874
[2] J. Jung, T. Mori, C. Kobayashi, Y. Matsunaga, T. Yoda, M. Feig, and Y. Sugita, WIREs Comput. Mol. Sci., 5, 310-323 (2015). https://doi.org/10.1002/wcms.1220
[3] D. L. Mobley, J. P. Guthrie,J. Comput. Aided Mol. Des.28, 711-720 (2014). https://doi.org/10.1007/s10822-014-9747-x
[2] J. Jung, T. Mori, C. Kobayashi, Y. Matsunaga, T. Yoda, M. Feig, and Y. Sugita, WIREs Comput. Mol. Sci., 5, 310-323 (2015). https://doi.org/10.1002/wcms.1220
[3] D. L. Mobley, J. P. Guthrie,J. Comput. Aided Mol. Des.28, 711-720 (2014). https://doi.org/10.1007/s10822-014-9747-x
Details of analysis
Related information
Inquiries Regarding Products
Have questions about product implementation? Contact us today.