Evaluation of Solubility Parameters Using MD
For water, octane, benzene, ethanol, and methanol, solubility parameters (SP values) were evaluated from Full-Atomistic Molecular Dynamics (FAMD) results. Cohesive energy was calculated from the potential energy difference between isolated molecules in pseudo-vacuum and bulk states, and SP values were estimated using J-OCTA’s DPD modeler function. Good agreement was obtained for both density and SP values.
Use Cases Highlights
- Estimation of SP values by MD
- Easy estimation using J-OCTA’s modeling functions
- Good agreement with experimental results
Estimation of SP values by MD
Solubility parameter (SP) values of water, octane, benzene, methanol, and ethanol from Full-Atomistic Molecular Dynamics (FAMD) are compared with experimental values, showing good agreement.
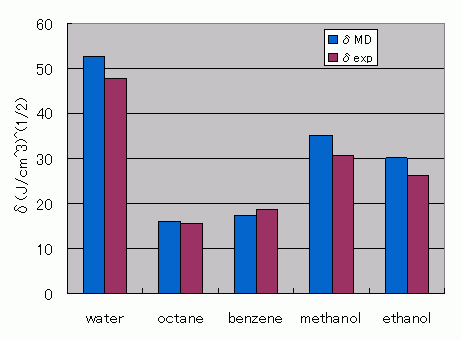
Comparison with experimental values
Reference
[1] A.Maiti and S. McGrother, J. Chem. Phys., 120, 3, 15 (2004)
[2] Allan F. M. Barton The CRC Handbook of Solubility Parameters and Other Cohesion Parameters, Second Edition CRC Press, (1991)
[2] Allan F. M. Barton The CRC Handbook of Solubility Parameters and Other Cohesion Parameters, Second Edition CRC Press, (1991)
Details of analysis
Inquiries Regarding Products
Have questions about product implementation? Contact us today.