Evaluation of Relative Permittivity Using MD
Full-Atomistic Molecular Dynamics (FAMD) calculations were used to evaluate the relative permittivity of water, octane, benzene, methanol, and ethanol. After equilibration at 300 K with VSOP, relative permittivity was calculated from time fluctuations of the dipole moment. Comparisons with experimental values clarified the effects of molecular structure on dielectric constant.
Use Cases Highlights
- Evaluation of dielectric constant using Full-Atomistic MD
- Agreement with experimental values
- Evaluation of the effect of molecular structure
Comparison of relative permittivity
Comparison between dielectric constants predicted by MD and experimental values is shown. High dielectric constants are obtained for water and methanol, and low for benzene and octane.
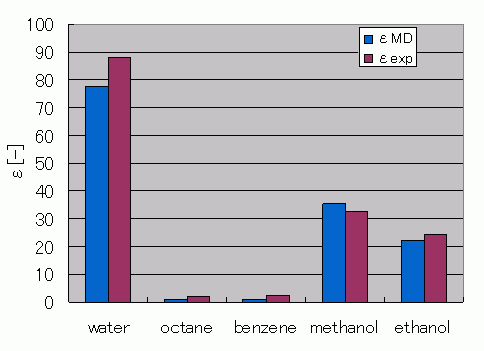
Comparison with experimental values
Reference
[1] N. Yoshii, S. Miura, S. Okazaki, Chem. Phys. Lett. 345 (2001) 195-200
[2] Chronological Scientific Tables 2005 Maruzen
[3] Chemistry Handbook 2004 Maruzen
[2] Chronological Scientific Tables 2005 Maruzen
[3] Chemistry Handbook 2004 Maruzen
Details of analysis
Inquiries Regarding Products
Have questions about product implementation? Contact us today.