Evaluation of Relative Permittivity Using MD and MO/DFT
The relative permittivity of substances such as benzene, acetone, and PVC was evaluated using Molecular Dynamics (MD) and quantum chemical calculations (MO/DFT). In MD, orientational polarization was calculated from time fluctuations of the dipole moment, while in MO/DFT, electronic polarization was calculated from polarizability. Comparisons with experimental values clarified contributions to dielectric constant depending on molecular structure.
Use Cases Highlights
- Evaluation of relative permittivity for various molecules
- Analysis enabling understanding of the origin of dielectric constant
- Applicability to polymers
Evaluation of relative permittivity of various molecules
Models of benzene and acetone for evaluating dielectric constant using MD and MO/DFT are shown.
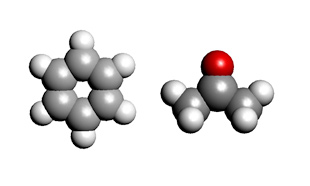
Analysis models (left: benzene, right: acetone)
Analysis enabling understanding of the origin of dielectric constant
Results of relative permittivity calculated for benzene and acetone using MO and MD are shown. MD used the OPLS force field, while MO used molecular polarizability calculated by Gaussian.
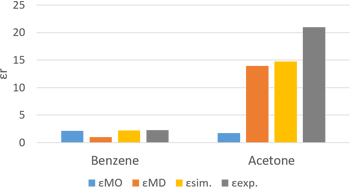
Calculated relative permittivity results
Applicability to polymers
An analysis model for evaluating the relative permittivity of PVC polymers by MD and QSPR is shown. In MD, a 100-molecule system was used, and in QSPR, the value was estimated from density.
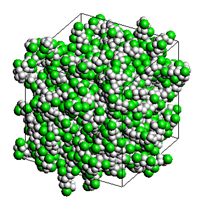
Analysis model (PVC)
Reference
[1] J.Bicerano, Prediction of Polymer Properties, 3rd Ed. Marcel Dekker, 2002
[2] J. Chem. Eng. Data 2018, 63, 5, 1170
[2] J. Chem. Eng. Data 2018, 63, 5, 1170
Details of analysis
Inquiries Regarding Products
Have questions about product implementation? Contact us today.